 In general, thermoluminescence is a form of luminescence. It is exhibited by certain crystalline materials, such as calcium fluoride, lithium fluoride, calcium sulfate, lithium borate, calcium borate, potassium bromide, and feldspar. Previously absorbed energy from electromagnetic radiation or other ionizing radiation in these materials is re-emitted as light upon heating of the material. The material must also be transparent to its own light emissions.
In general, thermoluminescence is a form of luminescence. It is exhibited by certain crystalline materials, such as calcium fluoride, lithium fluoride, calcium sulfate, lithium borate, calcium borate, potassium bromide, and feldspar. Previously absorbed energy from electromagnetic radiation or other ionizing radiation in these materials is re-emitted as light upon heating of the material. The material must also be transparent to its own light emissions.
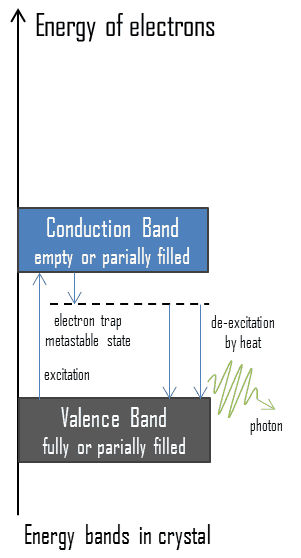
Electrons in some solids can exist in two energy states, called the valence band and the conduction band. The energy gap or the band gap is an energy range between valence band and conduction band where electron states are forbidden. The valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. In thermoluminescent materials, electrons may reach the conduction band, when they are excited, for example, by ionizing radiation (i.e. they must obtain energy higher than Egap). But in this case, defects in the material exist or impurities are added to trap electrons in the band gap and hold them there. These trapped electrons represent stored energy for the time that the electrons are held. This energy is given up if the electron returns to the valence band. When such crystals subsequently are heated the trapped electrons receive enough energy to escape from the trap and fall to the ground state. A portion of energy is emitted as light photons and a portion of energy is released as heat. Since warming is a requirement for this type of luminescence, the technique is called thermoluminescence.
Materials – Thermoluminescence
The two most common types of thermoluminescent materials used for dosimetry are calcium fluoride and lithium fluoride, with one or more impurities (e.g. manganese or magnesium) to produce trap states for energetic electrons. The impurity causes traps in the crystalline lattice where, following irradiation, electrons are held. When the crystal is warmed, the trapped electrons are released and light is emitted. The amount of light is related to the dose of radiation received by the crystal.
Calcium fluoride TLD is used to record gamma exposure, while lithium fluoride TLD is used for gamma and neutron exposure (indirectly, using the Li-6 (n,alpha)) nuclear reaction. Small crystals of LiF (lithium fluoride) are the most common TLD dosimeters since they have the same absorption properties as soft tissue. Lithium has two stable isotopes, lithium-6 (7.4 %) and lithium-7 (92.6 %). Li-6 is the isotope sensitive to neutrons. In order to record neutrons, LiF crystal dosimeters may be enriched in lithium-6 to enhance the lithium-6 (n,alpha) nuclear reaction.
We hope, this article, Thermoluminescence, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.